Analysis Notebook
Contents
2.22. Analysis Notebook#
A copy of this notebook is run to analyse the molecular dynamics simulations. The type of MD simulation is specified in the Snakemake rule as a parameter, such that it is accessible via: snakemake.params.method.
There are various additional analysis steps, that are included in the notebook,
but are not part of the paper. To turn these on, set the beta_run parameter to True.
There are also some commented out lines in the notebook. These are mainly for the purpose of debugging. Some of them are for interactively exploring the 3d structure of the system. These don’t work as part of the automated snakemake workflow, but can be enabled when running a notebook interactively.
#Check if we should use shortened trajectories for analysis.
if snakemake.config["shortened"]:
print("Using shortened trajectories and dihedrals. This only works if these were created previously!")
if not (os.path.exists(snakemake.params.traj_short)
and os.path.exists(snakemake.params.dihedrals_short)
and os.path.exists(snakemake.params.dPCA_weights_MC_short)
and os.path.exists(snakemake.params.weights_short)
):
raise FileNotFoundError("Shortened trajectories and dihedrals files do not exist, but config value is set to use shortened files! Switch off the use of shortenend files and first analyse this simulation using the full trajectory!")
else:
use_shortened = True
else:
use_shortened = False
# Imports
import matplotlib
import mdtraj as md
import numpy as np
import matplotlib.pyplot as plt
import matplotlib.tri as tri
import matplotlib.image as mpimg
# set matplotlib font sizes
SMALL_SIZE = 9
MEDIUM_SIZE = 11
BIGGER_SIZE = 13
plt.rc('font', size=MEDIUM_SIZE) # controls default text sizes
plt.rc('axes', titlesize=BIGGER_SIZE) # fontsize of the axes title
plt.rc('axes', labelsize=MEDIUM_SIZE) # fontsize of the x and y labels
plt.rc('xtick', labelsize=SMALL_SIZE) # fontsize of the tick labels
plt.rc('ytick', labelsize=SMALL_SIZE) # fontsize of the tick labels
plt.rc('legend', fontsize=MEDIUM_SIZE) # legend fontsize
plt.rc('figure', titlesize=BIGGER_SIZE) # fontsize of the figure title
DPI = 600
import scipy.cluster.hierarchy
from scipy.spatial.distance import squareform
import pandas as pd
sys.path.append(os.getcwd())
import src.dihedrals
import src.pca
import src.noe
import src.Ring_Analysis
import src.stats
from src.pyreweight import reweight
from src.utils import json_load, pickle_dump
from sklearn.manifold import TSNE
from sklearn.cluster import DBSCAN
from sklearn.neighbors import NearestNeighbors
from sklearn.decomposition import PCA
import nglview as nv
from rdkit import Chem
from rdkit.Chem.Draw import IPythonConsole
import py_rdl
import seaborn as sns
IPythonConsole.molSize = (900, 300) # (450, 150)
IPythonConsole.drawOptions.addStereoAnnotation = True
IPythonConsole.drawOptions.annotationFontScale = 1.5
import tempfile
import io
import svgutils.transform as sg
import svgutils.compose as sc
import scipy.stats as stats
from IPython.display import display, Markdown
# Can set a stride to make prelim. analysis faster. for production, use 1 (use all MD frames)
stride = int(snakemake.config["stride"])
print(f"Using stride {stride} to analyse MD simulations.")
# Perform additional analysis steps (e.g. compute structural digits)
beta_run = False
# Analysing compound
compound_index = int(snakemake.wildcards.compound_dir)
simtime = float(snakemake.wildcards.time)
# Storage for overview figure
final_figure_axs = []
Using stride 1 to analyse MD simulations.
2.22.1. Compound details#
display(Markdown(f"This notebook refers to compound {compound_index}."))
compound = json_load(snakemake.input.parm)
multi = compound.multi
if multi:
display(Markdown(
"According to the literature reference, there are two distinct structures in solution."
))
else:
display(Markdown(
"According to the literature reference, there is only one distinct structure in solution."
))
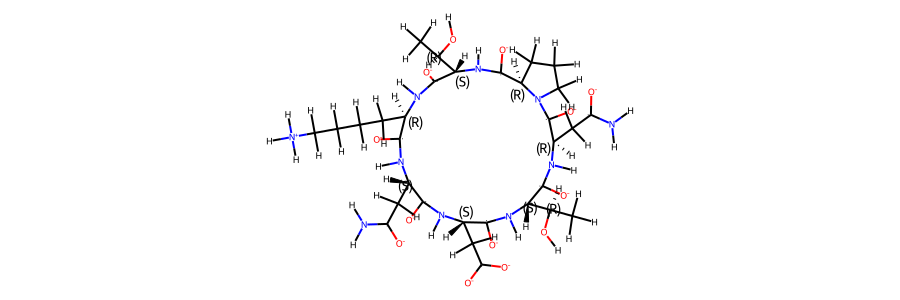
display(Markdown(f"""The sequence of the compound is **{compound.sequence}**. \n
A 2d structure of the compound is shown below."""))
This notebook refers to compound 57.
According to the literature reference, there is only one distinct structure in solution.
The sequence of the compound is T(DLY)NDT(DSG)(DPR).
A 2d structure of the compound is shown below.
2.22.2. Simulation details#
# TODO: change notebook that it supports use of a shortened trajectory file
# only load protein topology
topo = md.load_frame(snakemake.input.traj, 0, top=snakemake.input.top)
protein = topo.topology.select("protein or resname ASH")
display(Markdown(f"The following atom numbers are part of the protein: {protein}"))
The following atom numbers are part of the protein: [ 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103]
# Stereo check 1-frame trajectory to tmp-pdb file
t_stereo_check = topo.restrict_atoms(topo.topology.select("protein or resname ASH"))
tf = tempfile.NamedTemporaryFile(delete=False)
# tf.name
t_stereo_check.save_pdb(tf.name)
# Get reference mol
mol_ref = Chem.MolFromMol2File(
snakemake.input.ref_mol,
removeHs=False,
)
# Get 1st frame pdb from tempfile
post_eq_mol = Chem.MolFromPDBFile(
tf.name,
removeHs=False,
sanitize=False,
)
# could compare smiles to automate the stereo-check. Problem: mol2 reference file has wrong bond orders
# (amber does not write those correctly). The ref-pdb file cannot be read b/c geometry is not optimized.
# This leads to funky valences in rdkit. The post-eq pdb file reads fine but then charges etc. dont match
# with the reference (b/c of wrong bond orders). But can manually check that all stereocentres are correct (below)
Chem.CanonSmiles(Chem.MolToSmiles(post_eq_mol)) == Chem.CanonSmiles(
Chem.MolToSmiles(mol_ref)
)
display(Markdown("""Following we compare an annotated 2d structure of the compound's starting topology, with the
topology post equilibration"""))
Following we compare an annotated 2d structure of the compound's starting topology, with the topology post equilibration
post_eq_mol.RemoveAllConformers()
display(Markdown("2d structure of the compound post equilibration:"))
post_eq_mol
2d structure of the compound post equilibration:
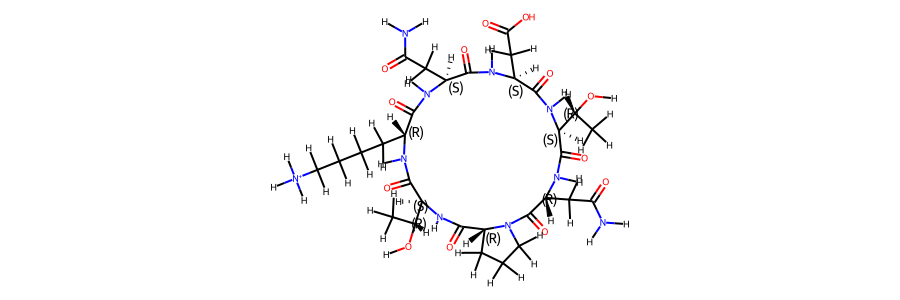
mol_ref.RemoveAllConformers()
display(Markdown("2d structure of the compound reference topology:"))
mol_ref
2d structure of the compound reference topology:
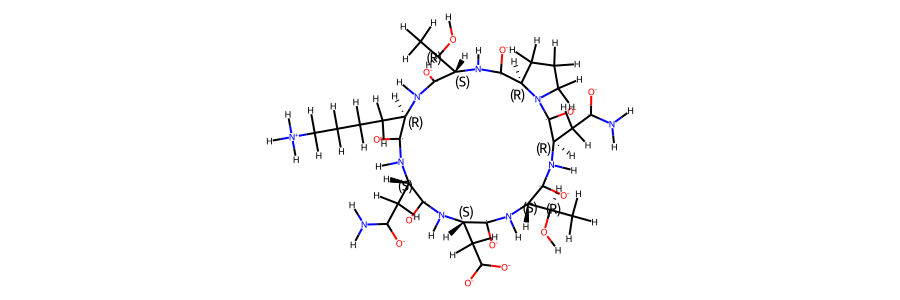
# load trajectory
display(Markdown("Now we load the MD trajectory."))
if not use_shortened:
t = md.load(
snakemake.input.traj, top=snakemake.input.top, atom_indices=protein, stride=stride
) # added strideint for GaMD 2k
print(t)
# Remove solvent from trajectory
t = t.restrict_atoms(t.topology.select("protein or resname ASH"))
t = t.superpose(t, 0)
# for GaMD, skip equlibration...
if snakemake.params.method == "GaMD":
weight_lengths = np.loadtxt(snakemake.input.weights)
weight_lengths = int(len(weight_lengths))
frames_start = int(t.n_frames - weight_lengths)
t = t[int(frames_start / stride) :] # added 13000 instead of 26000 for 2k
else:
frames_start = 0
print(t)
else:
stride = 1 # set stride to 1 for shortened files!
t = md.load(
snakemake.params.traj_short, top=snakemake.input.top, atom_indices=protein, stride=1
) # added strideint for GaMD 2k
t = t.restrict_atoms(t.topology.select("protein or resname ASH"))
t = t.superpose(t, 0)
Now we load the MD trajectory.
<mdtraj.Trajectory with 500000 frames, 104 atoms, 7 residues, and unitcells>
<mdtraj.Trajectory with 500000 frames, 104 atoms, 7 residues, and unitcells>
The simulation type is cMD, 2000 ns. The simulation was performed in H2O.
There are a total of 500000 frames available to analyse.
# Create a short trajectory & weights if working with the full trajectory
if not use_shortened:
# determine stride to get 10k frames:
stride_short = int(t.n_frames / 10000)
if stride_short == 0:
stride_short = 1
# save short trajectory to file
t[::stride_short].save_netcdf(snakemake.params.traj_short)
# load weights for GaMD
if snakemake.params.method != "cMD":
weight_data = np.loadtxt(snakemake.input.weights)
weight_data = weight_data[::stride]
#create shortened weights
np.savetxt(snakemake.params.weights_short, weight_data[::stride_short])
else:
# load shortened weights for GaMD
if snakemake.params.method != "cMD":
weight_data = np.loadtxt(snakemake.params.weights_short)
# this determines a cutoff for when we consider cis/trans conformers separately.
# only relevant if 2 sets of NOE values present.
# t.n_frames / 1000 -> 0.1% of frames need to be cis/trans to consider both forms.
CIS_TRANS_CUTOFF = int(t.n_frames / 1000)
However, for some of the analysis steps below, only 1% of these frames have been used to ensure better rendering in the browser.
2.22.3. Convergence of the simulation#
2.22.3.1. RMSD#
To check for convergence of the simulation, we can look at the root mean squared deviation of the atomic positions over the course of the simulation.
# compute rmsd for different atom types
rmsds = md.rmsd(t, t, 0) * 10
bo = topo.topology.select("protein and (backbone and name O)")
ca = topo.topology.select("name CA")
rmsds_ca = md.rmsd(t, t, 0, atom_indices=ca) * 10 # Convert to Angstrom!
rmsds_bo = md.rmsd(t, t, 0, atom_indices=bo) * 10 # Convert to Angstrom!
rmsds = rmsds[::100]
rmsds_ca = rmsds_ca[::100]
rmsds_bo = rmsds_bo[::100]
# Create x data (simulation time)
x = [x / len(rmsds_ca) * simtime for x in range(0, len(rmsds_ca))]
# Make plot
fig = figure(
plot_width=600,
plot_height=400,
title="RMSD of different atom types",
x_axis_label="Simulation time in ns",
y_axis_label="RMSD in angstrom, relative to first frame",
sizing_mode="stretch_width",
toolbar_location=None,
)
fig.line(
x,
rmsds,
line_width=2,
line_alpha=0.6,
legend_label="all atoms",
color="black",
muted_alpha=0.1,
)
fig.line(
x,
rmsds_ca,
line_width=2,
line_alpha=0.6,
legend_label="C-alpha atoms",
color="blue",
muted_alpha=0.1,
)
fig.line(
x,
rmsds_bo,
line_width=2,
line_alpha=0.6,
legend_label="backbone O atoms",
color="orange",
muted_alpha=0.1,
)
fig.legend.click_policy = "mute" #'hide'
show(fig)
# TODO: save rmsds as png, instead of manual screenshot https://docs.bokeh.org/en/latest/docs/user_guide/export.html
2.22.3.2. Dihedral angles#
if multi is not None:
multi = {v: k for k, v in multi.items()}
multiple = True
distinction = compound.distinction
print("Multiple compounds detected")
else:
multiple = False
pickle_dump(snakemake.output.multiple, multiple)
if multiple: # if Compound.cistrans:
ca_c = t.top.select(f"resid {distinction[0]} and name CA C")
n_ca_next = t.top.select(f"resid {distinction[1]} and name N CA")
omega = np.append(ca_c, n_ca_next)
t_omega_rad = md.compute_dihedrals(t, [omega])
t_omega_deg = np.abs(np.degrees(t_omega_rad))
plt.plot(t_omega_deg)
plt.hlines(90, 0, t.n_frames, color="red")
plt.xlabel("Frames")
plt.ylabel("Omega 0-1 [°]")
plt.title(f"Dihedral angle over time. Compound {compound_index}")
cis = np.where(t_omega_deg <= 90)[0]
trans = np.where(t_omega_deg > 90)[0]
pickle_dump(snakemake.output.multiple, (cis, trans))
# t[trans]
# TODO: save dihedrals as png
resnames = []
for i in range(0, t.n_residues):
resnames.append(t.topology.residue(i))
*_, omega = src.dihedrals.getDihedrals(t)
omega_deg = np.abs(np.degrees(omega))
omega_deg = omega_deg[::100]
simtime = float(snakemake.wildcards.time)
colors = src.utils.color_cycle()
# Create x data (simulation time)
x = [x / len(omega_deg) * simtime for x in range(0, len(omega_deg))]
# Make plot
fig = figure(
plot_width=600,
plot_height=400,
title="Omega dihedral angles over time",
x_axis_label="Simulation time in ns",
y_axis_label="Dihedral angle in ˚",
sizing_mode="stretch_width",
toolbar_location=None,
)
for res, i, col in zip(resnames, range(len(resnames)), colors):
fig.line(
x,
omega_deg[:, i],
line_width=2,
line_alpha=0.6,
legend_label=str(res),
color=col,
muted_alpha=0.1,
)
fig.legend.click_policy = "mute" #'hide'
show(fig)
# Compute dihedral angles [Phi] [Psi] [Omega]
phi, psi, omega = src.dihedrals.getDihedrals(t)
if beta_run:
# Print mean of dihedral angles [Phi] [Psi] [Omega]
print(
np.degrees(src.dihedrals.angle_mean(phi)),
np.degrees(src.dihedrals.angle_mean(psi)),
np.degrees(src.dihedrals.angle_mean(omega)),
)
# Plot ramachandran plot for each amino acid
if beta_run:
fig, axs = plt.subplots(int(np.ceil(len(phi.T) / 5)), 5, sharex="all", sharey="all")
fig.set_size_inches(16, 4)
motives = []
i = 0
for phi_i, psi_i in zip(np.degrees(phi.T), np.degrees(psi.T)):
weights_phi_psi = reweight(
np.column_stack((phi_i, psi_i)),
None,
"amdweight_MC",
weight_data,
)
axs.flatten()[i].scatter(
phi_i, psi_i, s=0.5, c=weights_phi_psi, vmin=0, vmax=8, cmap="Spectral_r"
)
axs.flatten()[i].set_title(i)
motives.append(src.dihedrals.miao_ramachandran(phi_i, psi_i))
i += 1
fig.show()
if beta_run:
# compute most common motives
combined_motives = np.column_stack((motives))
combined_motives = ["".join(test) for test in combined_motives]
from collections import Counter
c = Counter(combined_motives)
motive_percentage = [
(i, c[i] / len(combined_motives) * 100.0) for i, count in c.most_common()
]
# 10 most common motives and percentage of frames
print(motive_percentage[:10])
if beta_run:
# Get indicies of most common motives
combined_motives = np.array(combined_motives)
idxs = []
values = [i[0] for i in c.most_common(10)]
for i, v in enumerate(values):
idxs.append(np.where(combined_motives == v)[0])
2.22.4. Dimensionality Reductions#
The simulation trajectories contain the positions of all atoms. This high dimensional data (3*N_atoms) is too complicated to analyse by itself. To get a feeling of the potential energy landscape we need to apply some kind of dimensionality reduction. Here, we apply the PCA (Principal Component Analysis) method.
2.22.4.1. Cartesian PCA#
Details about cartesian PCA
c_pca, reduced_cartesian = src.pca.make_PCA(t, "cartesian")
# reweighting:
if snakemake.params.method == "cMD":
c_weights = reweight(reduced_cartesian, None, "noweight")
else:
c_weights = reweight(
reduced_cartesian, None, "amdweight_MC", weight_data
)
if multiple:
fig, axs = plt.subplots(1, 2, sharex="all", sharey="all", figsize=(6.7323, 3.2677))
axs[0] = src.pca.plot_PCA(
reduced_cartesian,
"cartesian",
compound_index,
c_weights,
"Energy [kcal/mol]",
fig,
axs[0],
explained_variance=c_pca.explained_variance_ratio_[:2],
)
axs[1] = src.pca.plot_PCA_citra(
reduced_cartesian[cis],
reduced_cartesian[trans],
"cartesian",
compound_index,
[multi["cis"] + " (cis)", multi["trans"] + " (trans)"],
fig,
axs[1],
)
else:
fig, ax = plt.subplots(figsize=(3.2677, 3.2677))
ax = src.pca.plot_PCA(
reduced_cartesian,
"cartesian",
compound_index,
c_weights,
"Energy [kcal/mol]",
fig,
ax,
explained_variance=c_pca.explained_variance_ratio_[:2],
)
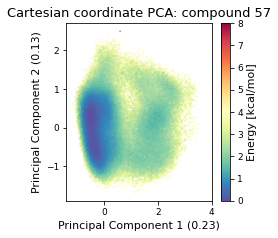
2.22.4.2. Pairwise distances PCA#
pd_pca, reduced_pd = src.pca.make_PCA(t, "pairwise_N_O")
# reweighting:
if snakemake.params.method == "cMD":
p_weights = reweight(reduced_pd, None, "noweight")
else:
p_weights = reweight(
reduced_pd, None, "amdweight_MC", weight_data
)
if multiple:
fig, axs = plt.subplots(1, 2, sharex="all", sharey="all", figsize=(6.7323, 3.2677))
axs[0] = src.pca.plot_PCA(
reduced_pd,
"pairwise",
compound_index,
p_weights,
"Energy [kcal/mol]",
fig,
axs[0],
explained_variance=pd_pca.explained_variance_ratio_[:2],
)
axs[1] = src.pca.plot_PCA_citra(
reduced_pd[cis],
reduced_pd[trans],
"pairwise",
compound_index,
[multi["cis"] + " (cis)", multi["trans"] + " (trans)"],
fig,
axs[1],
)
else:
fig, ax = plt.subplots(figsize=(3.2677, 3.2677))
ax = src.pca.plot_PCA(
reduced_pd,
"pairwise",
compound_index,
p_weights,
"Energy [kcal/mol]",
fig,
ax,
explained_variance=pd_pca.explained_variance_ratio_[:2],
)
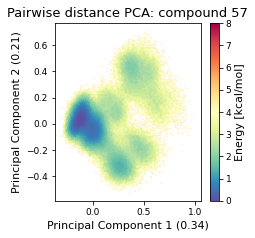
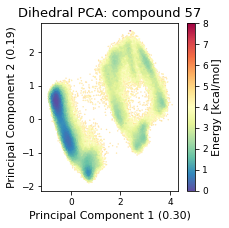
2.22.4.3. Dihedral PCA#
pca_d, reduced_dihedrals = src.pca.make_PCA(t, "dihedral")
reduced_dihedrals_full = src.dihedrals.getReducedDihedrals(t)
# save pca object & reduced dihedrals
pickle_dump(snakemake.output.dPCA, pca_d)
pickle_dump(snakemake.output.dihedrals, reduced_dihedrals_full)
if not use_shortened:
pickle_dump(snakemake.params.dihedrals_short, reduced_dihedrals_full[::stride_short])
# reweighting:
if snakemake.params.method == "cMD":
d_weights = reweight(reduced_dihedrals, None, "noweight")
else:
d_weights = reweight(
reduced_dihedrals, None, "amdweight_MC", weight_data
)
if multiple:
fig, axs = plt.subplots(1, 2, sharex="all", sharey="all", figsize=(6.7323, 3.2677))
axs[0] = src.pca.plot_PCA(
reduced_dihedrals,
"dihedral",
compound_index,
d_weights,
"Energy [kcal/mol]",
fig,
axs[0],
explained_variance=pca_d.explained_variance_ratio_[:2],
)
axs[1] = src.pca.plot_PCA_citra(
reduced_dihedrals[cis],
reduced_dihedrals[trans],
"dihedral",
compound_index,
[multi["cis"] + " (cis)", multi["trans"] + " (trans)"],
fig,
axs[1],
)
fig.savefig(snakemake.output.pca_dihe, dpi=DPI)
else:
fig, ax = plt.subplots(figsize=(3.2677, 3.2677))
ax = src.pca.plot_PCA(
reduced_dihedrals,
"dihedral",
compound_index,
d_weights,
"Energy [kcal/mol]",
fig,
ax,
explained_variance=pca_d.explained_variance_ratio_[:2],
)
fig.tight_layout()
fig.savefig(snakemake.output.pca_dihe, dpi=DPI)
final_figure_axs.append(sg.from_mpl(fig, savefig_kw={"dpi": DPI}))
pickle_dump(snakemake.output.dPCA_weights_MC, d_weights)
if not use_shortened:
pickle_dump(snakemake.params.dPCA_weights_MC_short, d_weights[::stride_short])
if beta_run:
# Plot structural digits on top of dPCA
fig, axs = plt.subplots(2, 5, sharex="all", sharey="all")
fig.set_size_inches(12, 8)
for i in range(10):
axs.flatten()[i] = src.pca.plot_PCA(
reduced_dihedrals,
"dihedral",
compound_index,
d_weights,
"Energy [kcal/mol]",
fig,
axs.flatten()[i],
cbar_plot="nocbar",
explained_variance=pca_d.explained_variance_ratio_[:2],
)
axs.flatten()[i].scatter(
reduced_dihedrals[idxs[i]][:, 0],
reduced_dihedrals[idxs[i]][:, 1],
label=values[i],
s=0.2,
marker=".",
color="black",
)
axs.flatten()[i].set_title(f"{values[i]}: {motive_percentage[i][1]:.2f}%")
fig.tight_layout()
2.22.4.4. TSNE#
# TSNE dimensionality reduction
# TSNE
if not use_shortened:
plot_stride = 100
else:
plot_stride = 1
cluster_stride = plot_stride # 125 previously
dihe = src.dihedrals.getReducedDihedrals(t)
tsne = TSNE(n_components=2, verbose=0, perplexity=50, n_iter=2000, random_state=42)
tsne_results = tsne.fit_transform(dihe[::cluster_stride, :]) # 250
plt.scatter(tsne_results[:, 0], tsne_results[:, 1])
plt.xlabel("t-SNE dimension 1")
plt.ylabel("t-SNE dimension 2")
plt.show()
/biggin/b147/univ4859/research/snakemake_conda/b998fbb8f687250126238eb7f5e2e52c/lib/python3.7/site-packages/sklearn/manifold/_t_sne.py:783: FutureWarning: The default initialization in TSNE will change from 'random' to 'pca' in 1.2.
FutureWarning,
/biggin/b147/univ4859/research/snakemake_conda/b998fbb8f687250126238eb7f5e2e52c/lib/python3.7/site-packages/sklearn/manifold/_t_sne.py:793: FutureWarning: The default learning rate in TSNE will change from 200.0 to 'auto' in 1.2.
FutureWarning,

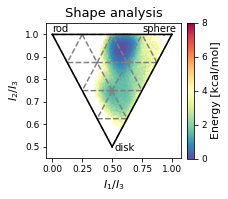
2.22.4.5. Shape analysis - principal moments of inertia#
inertia_tensor = md.compute_inertia_tensor(t)
principal_moments = np.linalg.eigvalsh(inertia_tensor)
# Compute normalized principal moments of inertia
npr1 = principal_moments[:, 0] / principal_moments[:, 2]
npr2 = principal_moments[:, 1] / principal_moments[:, 2]
mol_shape = np.stack((npr1, npr2), axis=1)
# Reweighting
if snakemake.params.method == "cMD":
mol_shape_weights = reweight(mol_shape, None, "noweight")
else:
mol_shape_weights = reweight(
mol_shape, None, "amdweight_MC", weight_data
)
# save
pickle_dump(snakemake.output.NPR_shape_data, mol_shape)
pickle_dump(snakemake.output.NPR_shape_weights, mol_shape_weights)
# Plot
x = mol_shape[:, 0]
y = mol_shape[:, 1]
v = mol_shape_weights
# create a triangulation out of these points
T = tri.Triangulation(x, y)
fig, ax = plt.subplots(figsize=(3.2677, 3.2677))
# plot the contour
# plt.tricontourf(x,y,T.triangles,v)
scat = ax.scatter(
mol_shape[:, 0],
mol_shape[:, 1],
s=0.5,
c=mol_shape_weights,
cmap="Spectral_r",
vmin=0,
vmax=8,
rasterized=True,
)
# create the grid
corners = np.array([[1, 1], [0.5, 0.5], [0, 1]])
triangle = tri.Triangulation(corners[:, 0], corners[:, 1])
# creating the outline
refiner = tri.UniformTriRefiner(triangle)
outline = refiner.refine_triangulation(subdiv=0)
# creating the outline
refiner = tri.UniformTriRefiner(triangle)
trimesh = refiner.refine_triangulation(subdiv=2)
# plotting the mesh
ax.triplot(trimesh, "--", color="grey")
ax.triplot(outline, "k-")
ax.set_xlabel(r"$I_{1}/I_{3}$")
ax.set_ylabel("$I_{2}/I_{3}$")
ax.text(0, 1.01, "rod")
ax.text(0.75, 1.01, "sphere")
ax.text(0.52, 0.48, "disk")
ax.set_ylim(0.45, 1.05) # 0.6
ax.set_xlim(-0.05, 1.08) # 1.13
ax.set_aspect(1.88) # 1.13 / 0.6
ax.set_title('Shape analysis')
colorbar = fig.colorbar(scat, label="Energy [kcal/mol]", fraction=0.046, pad=0.04)
fig.tight_layout()
fig.savefig(snakemake.output.NPR_shape_plot, dpi=DPI)
# final_figure_axs.append(sg.from_mpl(fig, savefig_kw={"dpi": DPI}))
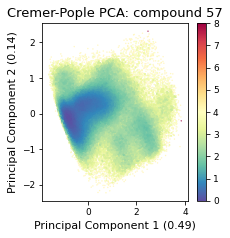
2.22.4.6. Cremer pople analysis#
# load topology reference
# mol_ref = Chem.MolFromPDBFile(pdb_amber, removeHs=False, proximityBonding=True) #removeHs=True, proximityBonding=True)
mol_ref = Chem.MolFromMol2File(
snakemake.input.ref_mol,
removeHs=False,
)
mol_ref.RemoveAllConformers()
display(Markdown("Topology Reference:"))
mol_ref
Topology Reference:
mol_ref.GetNumAtoms() == t.n_atoms
True
# Get Bond Set
bonds = []
for bond in mol_ref.GetBonds():
bonds.append((bond.GetBeginAtom().GetIdx(), bond.GetEndAtom().GetIdx()))
cremerpople_store = []
data = py_rdl.Calculator.get_calculated_result(bonds)
ring_length = []
for urf in data.urfs:
rcs = data.get_relevant_cycles_for_urf(urf)
for rc in rcs:
ring_length.append(
len(src.Ring_Analysis.Rearrangement(mol_ref, list(rc.nodes)))
)
max_ring = ring_length.index(max(ring_length))
# for urf in data.urfs:
urf = data.urfs[max_ring]
rcs = data.get_relevant_cycles_for_urf(urf)
for rc in rcs:
ringloop = src.Ring_Analysis.Rearrangement(
mol_ref, list(rc.nodes)
) # rearrange the ring atom order
# src.Ring_Analysis.CTPOrder(mol_ref, list(rc.nodes), n_res=t.n_residues) ## this does not work...
coord = t.xyz[:, ringloop]
for i in range(t.n_frames):
ccoord = src.Ring_Analysis.Translate(coord[i])
qs, angle = src.Ring_Analysis.GetRingPuckerCoords(
ccoord
) # get cremer-pople parameters
qs.extend([abs(x) for x in angle])
cremerpople_store.append(qs) # flatten tuple/list to just 1d list...
# coord = np.array([mol0.GetConformer(1).GetAtomPosition(atom) for atom in ringloop]) # get current ring atom coordinates
# ccoord = RA.Translate(coord) # translate ring with origin as cetner
# cremerpople = RA.GetRingPuckerCoords(ccoord) # get cremer-pople parameters
cremerpople_store = np.array(cremerpople_store)
# from sklearn.preprocessing import normalize
pca = PCA(n_components=2)
pca_input = cremerpople_store.reshape(t.n_frames, len(qs))
# normalize(cremerpople_store.reshape(t.n_frames, len(qs)))
cp_reduced_output = pca.fit_transform(pca_input)
if snakemake.params.method == "cMD":
cp_weights = reweight(cp_reduced_output, None, "noweight")
else:
cp_weights = reweight(
cp_reduced_output, None, "amdweight_MC", weight_data
)
ax = src.pca.plot_PCA(
cp_reduced_output,
"CP",
compound_index,
cp_weights,
explained_variance=pca.explained_variance_ratio_[:2],
)
if multiple:
src.pca.plot_PCA_citra(
cp_reduced_output[cis],
cp_reduced_output[trans],
"CP",
compound_index,
label=None,
fig=None,
ax=None,
)
2.22.4.7. Comparison#
# produce a shared datasource with shared labels
if not use_shortened:
plot_stride = 100
else:
plot_stride = 1
reduced_dihedrals_t = reduced_dihedrals[::plot_stride]
reduced_pd_t = reduced_pd[::plot_stride]
mol_shape_t = mol_shape[::plot_stride]
# Either show cremer pople, or show shapes
show_cremer_pople = False
if show_cremer_pople:
crepop_t = cp_reduced_output[::plot_stride]
tmp_dict = {
"dh_pc1": reduced_dihedrals_t[:, 0],
"dh_pc2": reduced_dihedrals_t[:, 1],
"pd_pc1": reduced_pd_t[:, 0],
"pd_pc2": reduced_pd_t[:, 1],
"tsne1": tsne_results[:, 0],
"tsne2": tsne_results[:, 1],
"cp1": crepop_t[:, 0],
"cp2": crepop_t[:, 1],
}
else:
tmp_dict = {
"dh_pc1": reduced_dihedrals_t[:, 0],
"dh_pc2": reduced_dihedrals_t[:, 1],
"pd_pc1": reduced_pd_t[:, 0],
"pd_pc2": reduced_pd_t[:, 1],
"tsne1": tsne_results[:, 0],
"tsne2": tsne_results[:, 1],
"npr1": mol_shape_t[:, 0],
"npr2": mol_shape_t[:, 1],
}
df = pd.DataFrame(tmp_dict)
source = ColumnDataSource(data=df)
# Linked plots in different representations
from bokeh.io import output_file, show
from bokeh.layouts import gridplot
from bokeh.models import ColumnDataSource, Label, LabelSet
from bokeh.plotting import figure
from bokeh.models import BooleanFilter, CDSView
TOOLS = "box_select,lasso_select,reset"
# create a new plot and add a renderer
left = figure(tools=TOOLS, plot_width=300, plot_height=300, title="Dihedral PCA")
left.dot("dh_pc1", "dh_pc2", source=source, selection_color="firebrick")
# create another new plot, add a renderer that uses the view of the data source
right = figure(
tools=TOOLS, plot_width=300, plot_height=300, title="Pairwise NO distances"
)
right.dot("pd_pc1", "pd_pc2", source=source, selection_color="firebrick")
rightr = figure(
tools=TOOLS, plot_width=300, plot_height=300, title="TSNE (of dihedral angles)"
)
rightr.dot("tsne1", "tsne2", source=source, selection_color="firebrick")
if show_cremer_pople:
rightrr = figure(tools=TOOLS, plot_width=300, plot_height=300, title="Cremer-Pople")
rightrr.dot("cp1", "cp2", source=source, selection_color="firebrick")
else:
rightrr = figure(tools=TOOLS, plot_width=300, plot_height=300, title="PMI")
rightrr.dot("npr1", "npr2", source=source, selection_color="firebrick")
rightrr.line([0.5, 0, 1, 0.5], [0.5, 1, 1, 0.5], line_width=2, color="black")
rightrr.line(
[0.45, -0.05, 1.05, 0.45],
[0.4, 1.1, 1.1, 0.4],
line_width=2,
color="white",
line_alpha=0,
)
triangle = ColumnDataSource(
data=dict(x=[0, 0.83, 0.44], y=[1, 1, 0.45], names=["rod", "sphere", "disk"])
)
labels = LabelSet(
x="x",
y="y",
text="names",
x_offset=0,
y_offset=0,
source=triangle,
render_mode="canvas",
)
rightrr.add_layout(labels)
p = gridplot([[left, right, rightr, rightrr]], sizing_mode="stretch_width")
show(p)
2.22.5. DBSCAN-Clustering#
The following section provides details about the performed DBSCAN clustering. Detailed plots about parameter derivation for the clustering are hidden, but can be revealed.
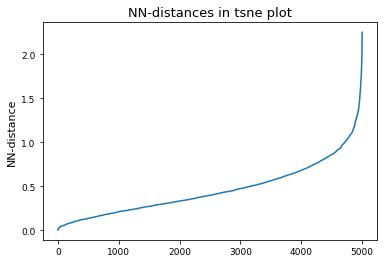
# Derive epsilon for DBSCAN-clustering from data: epsilon = max distance between nearest neighbors
nbrs = NearestNeighbors(n_neighbors=2).fit(tsne_results)
distances, indices = nbrs.kneighbors(tsne_results)
epsilon = distances.max()
distances = np.sort(distances, axis=0)
distances = distances[:, 1]
plt.plot(distances)
plt.title("NN-distances in tsne plot")
plt.ylabel("NN-distance")
plt.show()
# Perform DBSCAN-clustering with varying min_samples parameter
num_clusters = []
num_noise = []
for i in range(0, 200, 1):
clustering = DBSCAN(eps=epsilon, min_samples=i).fit(tsne_results)
labels = clustering.labels_
n_clusters = len(set(labels)) - (1 if -1 in labels else 0)
n_noise = list(labels).count(-1)
num_clusters.append(n_clusters)
num_noise.append(n_noise)
# Drop all points following the first detection of 0 clusters
num_clusters = np.array(num_clusters)
cutoff = np.argmin(num_clusters > 0)
num_clusters = num_clusters[:cutoff]
# print(num_clusters)
x = np.arange(0, len(num_clusters))
# Fit polynomial to detect right-most plateau
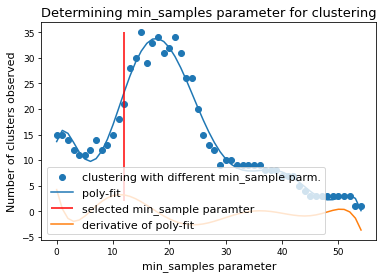
if x.size > 0:
mymodel = np.poly1d(np.polyfit(x, num_clusters, 8))
deriv = mymodel.deriv()
roots = deriv.roots
# discard complex roots
r_roots = roots[np.isreal(roots)].real
# discard negative values
r_roots = r_roots[r_roots >= 0]
# discard values greater than x.max()
r_roots = r_roots[r_roots <= x.max() - 3]
# Take largest root
if r_roots != []:
min_samples = int(r_roots.max())
print(f"min_samples = {min_samples} was selected as parameter for clustering")
else:
min_samples = 15
print(
"Caution! min samples parameter was selected as fixed value b/c automatic determination failed. specify the parameter manually in the config!"
)
else:
min_samples = 15
# If config overrides, use config value:
if snakemake.wildcards.index in snakemake.config["cluster_conf"]:
min_samples = int(snakemake.config["cluster_conf"][snakemake.wildcards.index])
print(
f"Override: Use min_samples={min_samples} instead of the above determined parameter"
)
min_samples = 48 was selected as parameter for clustering
/biggin/b147/univ4859/research/snakemake_conda/b998fbb8f687250126238eb7f5e2e52c/lib/python3.7/site-packages/ipykernel_launcher.py:18: DeprecationWarning: elementwise comparison failed; this will raise an error in the future.
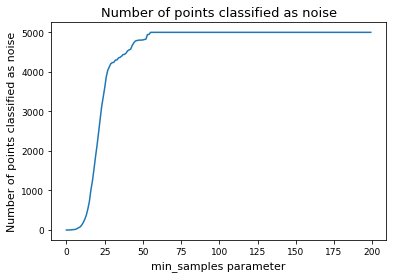
# Alternative: determine min_samples parameter by deciding for a fix fraction of points to classify as noise
# set cutoff as threshold percent of noise
threshhold = 0.05
num_noise = np.array(num_noise)
min_samples = np.argmin(num_noise < len(tsne_results) * threshhold)
# noise_cutoff
# min_samples = 8
display(f"Override: Use min_samples={min_samples} instead of the above determined parameter")
'Override: Use min_samples=12 instead of the above determined parameter'
plt.scatter(x, num_clusters, label="clustering with different min_sample parm.")
if x.size > 0:
plt.plot(x, mymodel(x), label="poly-fit")
plt.vlines(
min_samples,
2,
num_clusters.max(),
color="red",
label="selected min_sample paramter",
)
plt.plot(x, deriv(x), label="derivative of poly-fit")
plt.legend(loc="lower left")
plt.title("Determining min_samples parameter for clustering")
plt.xlabel("min_samples parameter")
plt.ylabel("Number of clusters observed")
plt.savefig(snakemake.output.cluster_min_samp, dpi=DPI)
plt.plot(num_noise, label="Number of points classified as noise")
plt.xlabel("min_samples parameter")
plt.ylabel("Number of points classified as noise")
plt.title("Number of points classified as noise")
plt.show()
# Perform clustering for selected min_samples parameter
clustering = DBSCAN(eps=epsilon, min_samples=min_samples).fit(tsne_results)
threshhold = 0.01 # 0.05
n_clusters = len(set(clustering.labels_)) - (1 if -1 in clustering.labels_ else 0)
print(f"There are {n_clusters} clusters")
cluster_points = []
cluster_label_filter = []
cluster_percentage = []
cluster_labels_sorted_by_population = list(dict.fromkeys(sorted(clustering.labels_, key=list(clustering.labels_).count, reverse=True)))
plt.figure(figsize=(3.2677, 3.2677))
for cluster in cluster_labels_sorted_by_population:
if cluster != -1:
if len(clustering.labels_[clustering.labels_ == cluster]) >= threshhold * len(
clustering.labels_
):
clus_points = tsne_results[clustering.labels_ == cluster]
plt.plot(
clus_points[:, 0],
clus_points[:, 1],
marker=".",
linewidth=0,
label=f"Cluster {cluster}",
)
percentage = len(clustering.labels_[clustering.labels_ == cluster]) / len(
clustering.labels_
)
plt.text(
x=np.mean(clus_points[:, 0]),
y=np.mean(clus_points[:, 1]),
s=f"{cluster}: {percentage*100:0.2f}%",
verticalalignment="center",
horizontalalignment="center",
)
print(
f"Cluster {cluster} makes up more than {threshhold * 100}% of points. ({percentage * 100:0.2f} % of total points)"
)
cluster_percentage.append(percentage)
cluster_points.append(clustering.labels_ == cluster)
cluster_label_filter.append(cluster)
else:
clus_points = tsne_results[clustering.labels_ == cluster]
plt.plot(
clus_points[:, 0],
clus_points[:, 1],
marker=".",
linewidth=0,
label=f"Cluster {cluster}",
alpha=0.1,
)
percentage = len(clustering.labels_[clustering.labels_ == cluster]) / len(
clustering.labels_
)
print(
f"Exlude Cluster {cluster} is less than {threshhold*100}% of points. ({percentage * 100:0.2f} % of total points)"
)
plt.plot
else:
clus_points = tsne_results[clustering.labels_ == cluster]
plt.plot(
clus_points[:, 0],
clus_points[:, 1],
marker=".",
linewidth=0,
label=f"Noise",
alpha=0.1,
color="grey",
)
percentage = len(clustering.labels_[clustering.labels_ == cluster]) / len(
clustering.labels_
)
print(f"Noise makes up {percentage * 100:0.2f} % of total points.")
# Shrink current axis by 20%
# plt.legend(loc="center right", bbox_to_anchor=(1.3,0.25))
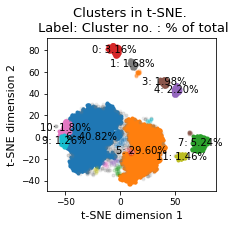
plt.title(f"Clusters in t-SNE. \n Label: Cluster no. : % of total")
plt.xlabel("t-SNE dimension 1")
plt.ylabel("t-SNE dimension 2")
plt.tight_layout()
plt.savefig(snakemake.output.cluster_plot, dpi=DPI)
There are 21 clusters
Cluster 6 makes up more than 1.0% of points. (40.82 % of total points)
Cluster 5 makes up more than 1.0% of points. (29.60 % of total points)
Noise makes up 5.38 % of total points.
Cluster 7 makes up more than 1.0% of points. (5.24 % of total points)
Cluster 0 makes up more than 1.0% of points. (3.16 % of total points)
Cluster 4 makes up more than 1.0% of points. (2.20 % of total points)
Cluster 3 makes up more than 1.0% of points. (1.98 % of total points)
Cluster 10 makes up more than 1.0% of points. (1.80 % of total points)
Cluster 1 makes up more than 1.0% of points. (1.68 % of total points)
Cluster 11 makes up more than 1.0% of points. (1.46 % of total points)
Cluster 9 makes up more than 1.0% of points. (1.26 % of total points)
Exlude Cluster 18 is less than 1.0% of points. (0.96 % of total points)
Exlude Cluster 2 is less than 1.0% of points. (0.90 % of total points)
Exlude Cluster 8 is less than 1.0% of points. (0.70 % of total points)
Exlude Cluster 12 is less than 1.0% of points. (0.54 % of total points)
Exlude Cluster 17 is less than 1.0% of points. (0.44 % of total points)
Exlude Cluster 16 is less than 1.0% of points. (0.44 % of total points)
Exlude Cluster 14 is less than 1.0% of points. (0.36 % of total points)
Exlude Cluster 19 is less than 1.0% of points. (0.34 % of total points)
Exlude Cluster 13 is less than 1.0% of points. (0.32 % of total points)
Exlude Cluster 20 is less than 1.0% of points. (0.30 % of total points)
Exlude Cluster 15 is less than 1.0% of points. (0.12 % of total points)
plt.figure(figsize=(3.2677, 3.2677))
plt.plot(clustering.labels_, marker=1, linewidth=0)
plt.title("Clusters over time (-1: noise)")
plt.xlabel("Snapshot")
plt.ylabel("Cluster no.")
plt.savefig(snakemake.output.cluster_time, dpi=DPI)

# Find cluster points in original trajectory, compute average structure,
# then find closest (min-rmsd) cluster structure to this
reduced_ind = np.arange(0, len(dihe), cluster_stride)
reduced_g_dihe = dihe[reduced_ind, :]
cluster_min_pca = []
cluster_index = []
mol_shape_cluster = []
t0 = t[0].time
dt = t.timestep
for i, cluster_name in zip(cluster_points, cluster_label_filter):
# cluster points in original trajectory
indices = reduced_ind[i]
avg_struct = np.mean(t[indices].xyz, axis=0)
avg_t = md.Trajectory(xyz=avg_struct, topology=None)
# compute average dihedral angles for each cluster:
phi, psi, omega = src.dihedrals.getDihedrals(t[indices])
print(
np.degrees(src.dihedrals.angle_mean(phi)),
np.degrees(src.dihedrals.angle_mean(psi)),
np.degrees(src.dihedrals.angle_mean(omega)),
)
# find min-RMSD structure to the average
rmsd = md.rmsd(t[indices], avg_t, 0)
min_rmsd_idx = np.where(rmsd == rmsd.min())
cluster_min = t[indices][min_rmsd_idx]
cluster_index.append(int((cluster_min.time - t0) / dt))
print(
f"Cluster {cluster_name}: Closest min structure is frame {int((cluster_min.time - t0) / dt)} (time: {float(cluster_min.time)})"
)
# Compute dihedrals of min-RMSD cluster structure, and transform to PCA
cluster_min = src.dihedrals.getReducedDihedrals(cluster_min)
cluster_min_pca.append(pca_d.transform(cluster_min))
# Compute shape
inertia_tensor_cluster = md.compute_inertia_tensor(t[indices][min_rmsd_idx])
principal_moments_cluster = np.linalg.eigvalsh(inertia_tensor_cluster)
# Compute normalized principal moments of inertia
npr1_cluster = principal_moments_cluster[:, 0] / principal_moments_cluster[:, 2]
npr2_cluster = principal_moments_cluster[:, 1] / principal_moments_cluster[:, 2]
mol_shape_cluster.append(np.stack((npr1_cluster, npr2_cluster), axis=1))
[-73.45704 132.61789 -80.187874 -61.388973 -90.69819 107.70024
60.26572 ] [ -2.7114425 19.418917 172.55582 -23.96031 -1.7248216
-129.87038 -131.71024 ] [ 177.68127 175.31357 174.67433 174.59827 -179.09975 176.9353
179.17046]
Cluster 6: Closest min structure is frame 150400 (time: 601604.0)
[-76.16969 73.68891 -82.05035 -62.74762 -89.86737 105.67758 59.09975] [ 50.533234 24.233116 170.5653 -25.8465 0.41487992
-137.17825 -133.73744 ] [ 177.44522 174.85202 174.58496 174.94565 -176.234 176.66058
178.15337]
Cluster 5: Closest min structure is frame 144900 (time: 579604.0)
[-115.61985 66.554665 -145.42395 -68.63933 -79.587944 122.2986
60.933174] [ 135.60214 22.562141 145.41649 -24.142185 -7.812761 -158.6108
-137.72243 ] [ 172.65996 -175.18681 -178.89014 177.60707 178.71838 -172.72256
175.60344]
Cluster 7: Closest min structure is frame 220400 (time: 881604.0)
[ -79.58769 127.64173 -112.640465 -72.580414 59.566727 85.62877
62.26685 ] [ 86.97455 -147.42148 -33.22059 116.150475 14.014114 -143.68495
-138.80072 ] [-176.1593 -176.21768 -178.75314 -174.65361 -178.03314 179.90396
175.50706]
Cluster 0: Closest min structure is frame 500 (time: 2004.0)
[-141.90143 71.35959 -121.84202 -72.20867 58.59354 96.487785
57.57126 ] [ 131.48624 3.25683 165.55234 148.79878 58.76849 -151.55865
-142.46768] [-178.5138 -178.96288 172.56918 176.4324 178.03087 -177.50917
171.88905]
Cluster 4: Closest min structure is frame 16000 (time: 64004.0)
[ -82.96243 86.62505 -101.95975 -58.713703 60.074127 123.53998
61.673573] [ 40.731632 32.94976 -175.0762 134.52112 12.590131 -125.42631
-141.9562 ] [-177.45744 -178.87234 167.90999 -176.63997 173.31372 -177.27072
176.6937 ]
Cluster 3: Closest min structure is frame 12700 (time: 50804.0)
[ -71.31772 141.01942 -124.6663 -59.93835 -91.73323 100.34749
61.78844] [ -11.915665 53.76541 173.23265 -24.68462 2.2759926
-122.61044 -139.3426 ] [-179.77507 -179.79875 177.45125 174.87184 -176.31566 176.94287
178.27797]
Cluster 10: Closest min structure is frame 471700 (time: 1886804.0)
[ -71.39583 128.42122 -105.30741 -87.96758 54.287964 115.28553
58.46625 ] [ -3.5853646 -36.362392 -11.342673 34.28735 48.960014
-145.32938 -127.5622 ] [ 177.15552 -177.04353 -177.45012 -165.76736 -163.93507 178.77489
177.84433]
Cluster 1: Closest min structure is frame 1100 (time: 4404.0)
[-101.03583 76.996376 -70.34961 -68.58019 -78.91795 126.80635
59.985703] [ 132.69235 -51.32609 147.2851 -21.07477 -8.79613 -161.81482
-143.15382] [ 175.88051 -174.97278 -179.20116 177.9012 178.39293 -172.18182
174.24753]
Cluster 11: Closest min structure is frame 142800 (time: 571204.0)
[ -70.38642 139.6927 -120.06013 -64.6888 -93.76059 131.67766
60.359375] [ -8.836196 52.834175 176.16963 -21.794445 -17.287973 -118.91924
-142.92043 ] [ 177.78247 177.03996 -176.0423 174.97147 179.82056 176.24612
175.62244]
Cluster 9: Closest min structure is frame 85800 (time: 343204.0)
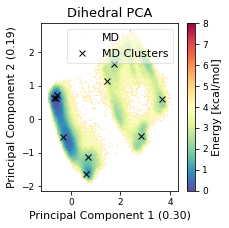
# Plot cluster mins in original d-PCA plot
fig, ax = plt.subplots(figsize=(3.2677, 3.2677))
ax = src.pca.plot_PCA(
reduced_dihedrals,
"dihedral",
compound_index,
d_weights,
"Energy [kcal/mol]",
fig,
ax,
explained_variance=pca_d.explained_variance_ratio_[:2],
)
# ADD LEGEND ENTRY FOR MD
ax.plot(
np.array(cluster_min_pca)[:, 0, 0],
np.array(cluster_min_pca)[:, 0, 1],
label="Clusters",
linewidth=0,
marker="x",
c='black',
)
ax.legend(["MD","MD Clusters"], framealpha=0.5)
fig.tight_layout()
ax.set_title("Dihedral PCA")
fig.savefig(snakemake.output.cluster_pca, dpi=DPI)
final_figure_axs.append(sg.from_mpl(fig, savefig_kw={"dpi": DPI}))
/biggin/b147/univ4859/research/snakemake_conda/b998fbb8f687250126238eb7f5e2e52c/lib/python3.7/site-packages/ipykernel_launcher.py:25: UserWarning: Creating legend with loc="best" can be slow with large amounts of data.
/biggin/b147/univ4859/research/snakemake_conda/b998fbb8f687250126238eb7f5e2e52c/lib/python3.7/site-packages/ipykernel_launcher.py:28: UserWarning: Creating legend with loc="best" can be slow with large amounts of data.
/biggin/b147/univ4859/research/snakemake_conda/b998fbb8f687250126238eb7f5e2e52c/lib/python3.7/site-packages/svgutils/transform.py:425: UserWarning: Creating legend with loc="best" can be slow with large amounts of data.
fig.savefig(fid, format="svg", **savefig_kw)
/biggin/b147/univ4859/research/snakemake_conda/b998fbb8f687250126238eb7f5e2e52c/lib/python3.7/site-packages/IPython/core/pylabtools.py:151: UserWarning: Creating legend with loc="best" can be slow with large amounts of data.
fig.canvas.print_figure(bytes_io, **kw)
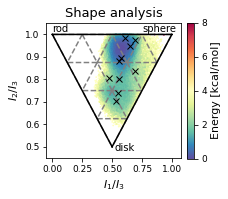
# Plot cluster mins in shape plot
# Plot
x = mol_shape[:, 0]
y = mol_shape[:, 1]
v = mol_shape_weights
# create a triangulation out of these points
T = tri.Triangulation(x, y)
fig, ax = plt.subplots(figsize=(3.2677, 3.2677))
# plot the contour
# plt.tricontourf(x,y,T.triangles,v)
scat = ax.scatter(
mol_shape[:, 0],
mol_shape[:, 1],
s=0.5,
c=mol_shape_weights,
cmap="Spectral_r",
vmin=0,
vmax=8,
rasterized=True,
)
# create the grid
corners = np.array([[1, 1], [0.5, 0.5], [0, 1]])
triangle = tri.Triangulation(corners[:, 0], corners[:, 1])
# creating the outline
refiner = tri.UniformTriRefiner(triangle)
outline = refiner.refine_triangulation(subdiv=0)
# creating the outline
refiner = tri.UniformTriRefiner(triangle)
trimesh = refiner.refine_triangulation(subdiv=2)
# plotting the mesh
ax.triplot(trimesh, "--", color="grey")
ax.triplot(outline, "k-")
ax.set_xlabel(r"$I_{1}/I_{3}$")
ax.set_ylabel("$I_{2}/I_{3}$")
ax.text(0, 1.01, "rod")
ax.text(0.75, 1.01, "sphere")
ax.text(0.52, 0.48, "disk")
ax.set_ylim(0.45, 1.05) # 0.6
ax.set_xlim(-0.05, 1.08) # 1.13
ax.set_aspect(1.88) # 1.13 / 0.6
ax.set_title('Shape analysis')
ax.plot(
np.array(mol_shape_cluster)[:, 0, 0],
np.array(mol_shape_cluster)[:, 0, 1],
label="Clusters",
linewidth=0,
marker="x",
c='black',
)
# ax.legend(["MD","MD Clusters"], framealpha=0.5)
colorbar = fig.colorbar(scat, label="Energy [kcal/mol]", fraction=0.046, pad=0.04)
fig.tight_layout()
fig.savefig(snakemake.output.NPR_shape_plot, dpi=DPI)
final_figure_axs.append(sg.from_mpl(fig, savefig_kw={"dpi": DPI}))
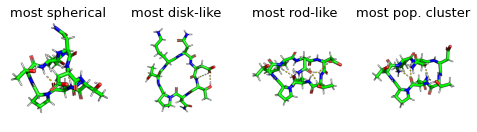
display(Markdown("Show most extreme structures."))
most_spherical = (x + y).argmax()
most_disk = (x - 0.5 + y - 0.5).argmin()
most_rod = (1 - y + x).argmin()
most_occupied_cluster = cluster_index[0]
fig, axs = plt.subplots(1,4, figsize=(6.7323, 3.2677 / 1.8))
for ax, frame_i, title_i in zip(axs.flatten(), [most_spherical, most_disk, most_rod, most_occupied_cluster], ["most spherical", "most disk-like", "most rod-like", "most pop. cluster"]):
img = src.utils.pymol_image(t[frame_i], t[most_occupied_cluster])
ax.imshow(img)
ax.set_title(title_i)
ax.axis('off')
fig.tight_layout()
# fig.subplots_adjust(wspace=0, hspace=0)
final_figure_axs.append(sg.from_mpl(fig, savefig_kw={"dpi": DPI}))
Show most extreme structures.
# create a new plot and add a renderer
plot_stride = 10
from bokeh.models import HoverTool
x = np.array(cluster_min_pca)[:, 0, 0]
y = np.array(cluster_min_pca)[:, 0, 1]
percentage = cluster_percentage
data = dict(x=x, y=y, percentage=percentage)
p = figure(
plot_width=400,
plot_height=400,
title="Average cluster structures in PCA space",
tools="reset",
)
p.dot(
reduced_dihedrals[::plot_stride, 0],
reduced_dihedrals[::plot_stride, 1],
selection_color="firebrick",
legend_label="simulation frames",
)
hoverable = p.triangle(
x="x", y="y", source=data, color="firebrick", size=8, legend_label="Clusters"
)
p.add_tools(
HoverTool(
tooltips=[("cluster #", "$index"), ("% of total frames", "@percentage{0.0%}")],
renderers=[hoverable],
)
)
show(p)
# Interactive viewing of clusters in 3d (needs running jupyter notebook)
cluster_traj = t[cluster_index]
cluster_traj.superpose(
cluster_traj, 0, atom_indices=cluster_traj.top.select("backbone")
)
view = nv.show_mdtraj(cluster_traj)
view
# save rst files from clusters, account for GaMD equilibration. Does not work with stride.
cluster_full_store = md.load_frame(snakemake.input.traj, cluster_index[0] + frames_start, top=snakemake.input.top)
for idx in cluster_index:
cluster_full_t = md.load_frame(snakemake.input.traj, idx, top=snakemake.input.top)
cluster_full_t.save_netcdfrst(
f"{snakemake.params.rst_dir}rst_{idx}.rst"
)
cluster_full_store = cluster_full_store.join(cluster_full_t, discard_overlapping_frames=True)
cluster_full_store.superpose(
cluster_full_store, 0, atom_indices=cluster_full_t.top.select("backbone")
)
cluster_full_store.save_pdb(snakemake.output.cluster_solvated)
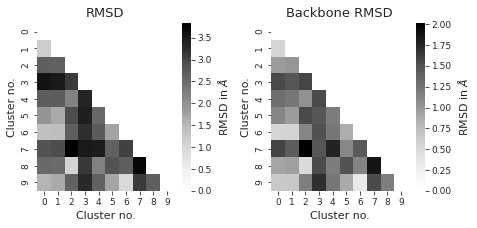
# compute rmsd between clusters
from itertools import combinations
indices = list(combinations(range(cluster_traj.n_frames), 2))
rmsd_backbone = np.zeros((cluster_traj.n_frames, cluster_traj.n_frames))
rmsd = np.zeros((cluster_traj.n_frames, cluster_traj.n_frames))
for i, j in indices:
rmsd_backbone[i, j] = (
md.rmsd(
cluster_traj[i],
cluster_traj[j],
atom_indices=cluster_traj.top.select("backbone"),
)
* 10
)
rmsd[i, j] = md.rmsd(cluster_traj[i], cluster_traj[j]) * 10
sns.set_style("ticks")
fig, axs = plt.subplots(1, 2, figsize=(6.7323, 3.2677))
titles = ["RMSD", "Backbone RMSD"] # between different clusters
for i, X in enumerate([rmsd, rmsd_backbone]):
X = X + X.T - np.diag(np.diag(X))
# get lower diagonal matrix
X = np.tril(X)
df = pd.DataFrame(X)
axs[i] = sns.heatmap(
df, annot=False, cmap="Greys", ax=axs[i], cbar_kws={"label": r"RMSD in $\AA$"}
)
axs[i].set_title(titles[i])
axs[i].set_xlabel("Cluster no.")
axs[i].set_ylabel("Cluster no.")
# ax.invert_xaxis()
fig.tight_layout()
plt.show()
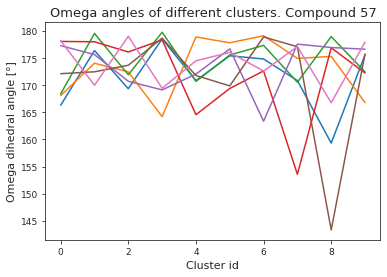
# compute dihedral angles
*_, omega = src.dihedrals.getDihedrals(cluster_traj)
omega_deg = np.abs(np.degrees(omega))
plt.plot(omega_deg)
plt.title(f"Omega angles of different clusters. Compound {compound_index}")
plt.xlabel("Cluster id")
plt.ylabel("Omega dihedral angle [°]")
plt.show()
pymol_script = f"""load {snakemake.output.cluster_pdb}
# inspired by: https://gist.github.com/bobbypaton/1cdc4784f3fc8374467bae5eb410edef
cmd.bg_color("white")
cmd.set("ray_opaque_background", "off")
cmd.set("orthoscopic", 0)
cmd.set("transparency", 0.1)
cmd.set("dash_gap", 0)
cmd.set("ray_trace_mode", 1)
cmd.set("ray_texture", 2)
cmd.set("antialias", 3)
cmd.set("ambient", 0.5)
cmd.set("spec_count", 5)
cmd.set("shininess", 50)
cmd.set("specular", 1)
cmd.set("reflect", .1)
cmd.space("cmyk")
#cmd.cartoon("oval")
cmd.show("sticks")
cmd.show("spheres")
cmd.color("gray85","elem C")
cmd.color("gray98","elem H")
cmd.color("slate","elem N")
cmd.color("red","elem O")
cmd.set("stick_radius",0.07)
cmd.set("sphere_scale",0.18)
cmd.set("sphere_scale",0.13, "elem H")
cmd.set("dash_gap",0.01)
cmd.set("dash_radius",0.07)
cmd.set("stick_color","black")
cmd.set("dash_gap",0.01)
cmd.set("dash_radius",0.035)
cmd.hide("nonbonded")
cmd.hide("cartoon")
cmd.hide("lines")
cmd.orient()
cmd.zoom()
cmd.hide("labels")
cmd.mpng("{snakemake.params.cluster_dir}test_", width=1000, height=1000)
"""
pymol_script_file = f"{snakemake.params.cluster_dir}pym.pml"
with open(pymol_script_file, "w") as f:
f.write(pymol_script)
# Run pymol to plot clusters
!pymol -c $pymol_script_file
PyMOL(TM) Molecular Graphics System, Version 2.5.0.
Copyright (c) Schrodinger, LLC.
All Rights Reserved.
Created by Warren L. DeLano, Ph.D.
PyMOL is user-supported open-source software. Although some versions
are freely available, PyMOL is not in the public domain.
If PyMOL is helpful in your work or study, then please volunteer
support for our ongoing efforts to create open and affordable scientific
software by purchasing a PyMOL Maintenance and/or Support subscription.
More information can be found at "http://www.pymol.org".
Enter "help" for a list of commands.
Enter "help <command-name>" for information on a specific command.
Hit ESC anytime to toggle between text and graphics.
Detected 24 CPU cores. Enabled multithreaded rendering.
PyMOL>load data/processed/refactor-test/results/57/H2O/cMD/2000/0/5dc9eaf6e041f3f2_clusters/clusters.pdb
ObjectMolecule: Read crystal symmetry information.
ObjectMoleculeReadPDBStr: read MODEL 1
ObjectMoleculeReadPDBStr: read MODEL 2
ObjectMoleculeReadPDBStr: read MODEL 3
ObjectMoleculeReadPDBStr: read MODEL 4
ObjectMoleculeReadPDBStr: read MODEL 5
ObjectMoleculeReadPDBStr: read MODEL 6
ObjectMoleculeReadPDBStr: read MODEL 7
ObjectMoleculeReadPDBStr: read MODEL 8
ObjectMoleculeReadPDBStr: read MODEL 9
ObjectMoleculeReadPDBStr: read MODEL 10
CmdLoad: "" loaded as "clusters".
PyMOL>cmd.bg_color("white")
PyMOL>cmd.set("ray_opaque_background", "off")
PyMOL>cmd.set("orthoscopic", 0)
PyMOL>cmd.set("transparency", 0.1)
PyMOL>cmd.set("dash_gap", 0)
PyMOL>cmd.set("ray_trace_mode", 1)
PyMOL>cmd.set("ray_texture", 2)
PyMOL>cmd.set("antialias", 3)
PyMOL>cmd.set("ambient", 0.5)
PyMOL>cmd.set("spec_count", 5)
PyMOL>cmd.set("shininess", 50)
PyMOL>cmd.set("specular", 1)
PyMOL>cmd.set("reflect", .1)
PyMOL>cmd.space("cmyk")
Color: loaded table '/biggin/b147/univ4859/research/snakemake_conda/b998fbb8f687250126238eb7f5e2e52c/lib/python3.7/site-packages/pymol/pymol_path/data/pymol/cmyk.png'.
PyMOL>cmd.show("sticks")
PyMOL>cmd.show("spheres")
PyMOL>cmd.color("gray85","elem C")
PyMOL>cmd.color("gray98","elem H")
PyMOL>cmd.color("slate","elem N")
PyMOL>cmd.color("red","elem O")
PyMOL>cmd.set("stick_radius",0.07)
PyMOL>cmd.set("sphere_scale",0.18)
PyMOL>cmd.set("sphere_scale",0.13, "elem H")
PyMOL>cmd.set("dash_gap",0.01)
PyMOL>cmd.set("dash_radius",0.07)
PyMOL>cmd.set("stick_color","black")
PyMOL>cmd.set("dash_gap",0.01)
PyMOL>cmd.set("dash_radius",0.035)
PyMOL>cmd.hide("nonbonded")
PyMOL>cmd.hide("cartoon")
PyMOL>cmd.hide("lines")
PyMOL>cmd.orient()
PyMOL>cmd.zoom()
PyMOL>cmd.hide("labels")
PyMOL>cmd.mpng("data/processed/refactor-test/results/57/H2O/cMD/2000/0/5dc9eaf6e041f3f2_clusters/test_", width=1000, height=1000)
Movie: frame 1 of 10, 0.87 sec. (0:00:08 - 0:00:08 to go).
Movie: frame 2 of 10, 0.87 sec. (0:00:07 - 0:00:07 to go).
Movie: frame 3 of 10, 0.88 sec. (0:00:07 - 0:00:06 to go).
Movie: frame 4 of 10, 0.87 sec. (0:00:06 - 0:00:06 to go).
Movie: frame 5 of 10, 0.89 sec. (0:00:05 - 0:00:05 to go).
Movie: frame 6 of 10, 0.88 sec. (0:00:04 - 0:00:04 to go).
Movie: frame 7 of 10, 0.86 sec. (0:00:03 - 0:00:03 to go).
Movie: frame 8 of 10, 0.85 sec. (0:00:02 - 0:00:02 to go).
Movie: frame 9 of 10, 0.89 sec. (0:00:01 - 0:00:01 to go).
Movie: frame 10 of 10, 0.86 sec. (0:00:00 - 0:00:00 to go).
data = []
cluster_imgs = [
f"{snakemake.params.cluster_dir}test_{str(i+1).zfill(4)}.png"
for i in range(cluster_traj.n_frames)
]
[data.append(mpimg.imread(img)) for img in cluster_imgs]
display("Pymol images read")
'Pymol images read'
# get default colors
colors = plt.rcParams["axes.prop_cycle"].by_key()["color"]
# make colors longer if more clusters than colors...
while len(cluster_label_filter) > len(colors):
colors.extend(colors)
print("Colors appended..")
fig, axs = plt.subplots(len(cluster_label_filter), 3, sharex="col", squeeze=False)
fig.set_size_inches(12, 3 * len(cluster_label_filter))
# plot cluster images
for i in range(cluster_traj.n_frames):
# print(f"final {i}")
axs[i, 0].imshow(data[i])
axs[i, 0].tick_params(
axis="both",
which="both",
bottom=False,
top=False,
left=False,
labelleft=False,
labelbottom=False,
)
# axs[i,0].tick_params(axis='y', which='both', bottom=False, top=False, labelbottom=False)
# plot corresponding pca's:
for i in range(cluster_traj.n_frames):
axs[i, 1].scatter(
reduced_dihedrals[:, 0],
reduced_dihedrals[:, 1],
marker=".",
s=0.5,
alpha=1,
c="black",
)
# add cluster representations
for ii, iii, iiii in zip(
cluster_min_pca, cluster_label_filter, range(len(cluster_label_filter))
):
(clus,) = axs[iiii, 1].plot(
ii[:, 0],
ii[:, 1],
marker="^",
label=f"Cluster {iii}",
linewidth=0,
c=colors[iiii],
)
# clus.get_color()
# add noe plots
for i, j, k in zip(range(cluster_traj.n_frames), cluster_index, cluster_label_filter):
NOE = src.noe.read_NOE(snakemake.input.noe)
if multiple:
NOE_trans, NOE_cis = NOE
NOE_cis_dict = NOE_cis.to_dict(orient="index")
NOE_trans_dict = NOE_trans.to_dict(orient="index")
else:
NOE_dict = NOE.to_dict(orient="index")
current_cluster = cluster_traj[i]
# print(j)
if multiple:
if j in cis:
# print("cis")
NOE_dict = NOE_cis_dict
NOE = NOE_cis
axs[i, 2].set_title(f"Cluster {k} (cis)")
else:
# print("trans!")
NOE_dict = NOE_trans_dict
NOE = NOE_trans
axs[i, 2].set_title(f"Cluster {k} (trans)")
else:
axs[i, 2].set_title(f"Cluster {k}")
NOE["md"], _, _2, NOE_dist, _3 = src.noe.compute_NOE_mdtraj(
NOE_dict, current_cluster
)
# Deal with ambigous NOEs
NOE = NOE.explode("md")
# and ambigous/multiple values
NOE = NOE.explode("NMR exp")
fig, axs[i, 2] = src.noe.plot_NOE(NOE, fig, axs[i, 2])
fig.tight_layout()
fig.savefig(snakemake.output.cluster_structs)
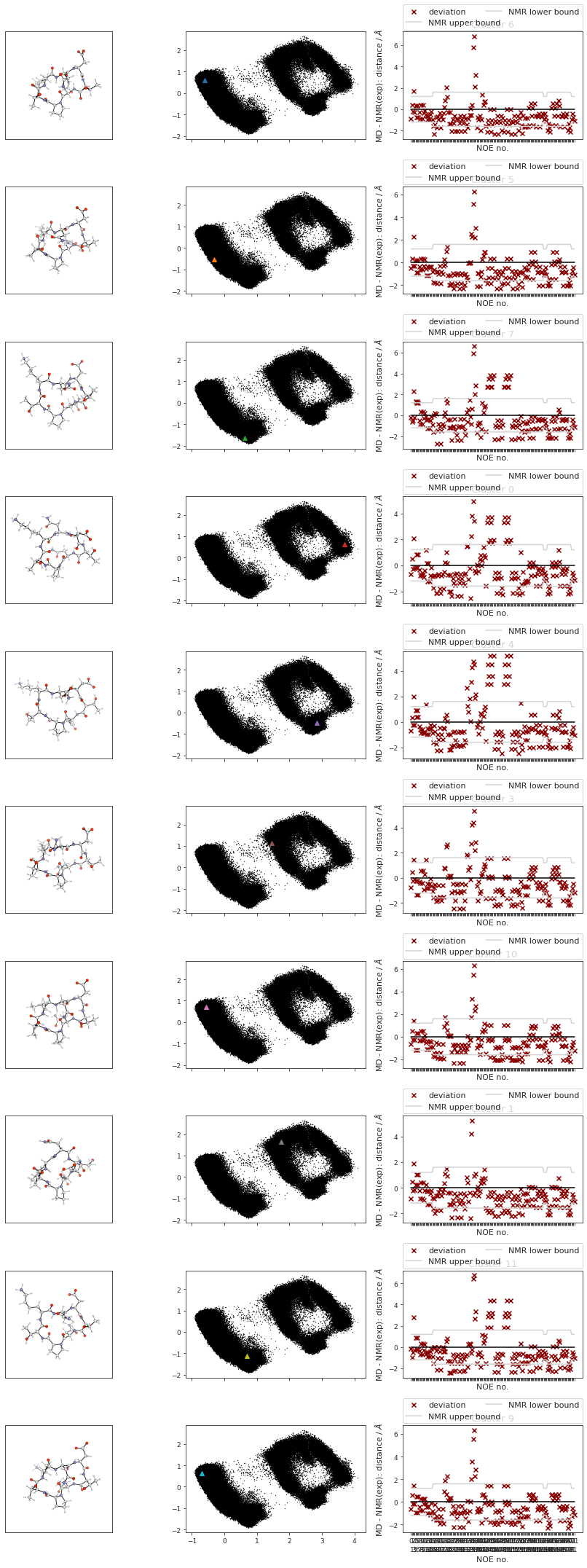
# TODO? cluster NOE statistics....
2.22.6. NOEs#
In the following section, we compute the NOE values for the simulation.
NOE = src.noe.read_NOE(snakemake.input.noe)
NOE_output = {}
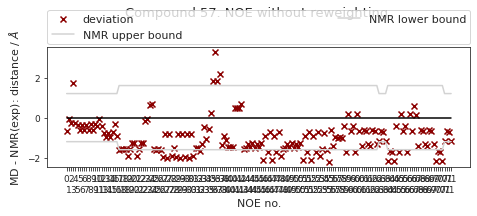
2.22.6.1. NOE without reweighting.#
The following NOE plot is computed via \(r^{-6}\) averaging. No reweighting is performed. (so unless the simulation is a conventional MD simulation, the following plot is not a valid comparison to experiment.)
if multiple:
fig, axs = plt.subplots(2, 1, figsize=(6.7323, 3.2677))
NOE_trans, NOE_cis = NOE
NOE_cis_dict = NOE_cis.to_dict(orient="index")
NOE_trans_dict = NOE_trans.to_dict(orient="index")
if len(cis) > CIS_TRANS_CUTOFF:
NOE_cis["md"], _, _2, NOE_dist_cis, _3 = src.noe.compute_NOE_mdtraj(
NOE_cis_dict, t[cis]
)
NOE_output[f"{multi['cis']}"] = NOE_cis.to_dict(orient="index")
# Deal with ambigous NOEs
NOE_cis = NOE_cis.explode("md")
# and ambigous/multiple values
NOE_cis = NOE_cis.explode("NMR exp")
fig, axs[1] = src.noe.plot_NOE(NOE_cis, fig, axs[1])
axs[1].set_title(f"Compound {multi['cis']} (cis)")
else:
print("Cis skipped because no frames are cis.")
if len(trans) > CIS_TRANS_CUTOFF:
NOE_trans["md"], _, _2, NOE_dist_trans, _3 = src.noe.compute_NOE_mdtraj(
NOE_trans_dict, t[trans]
)
NOE_output[f"{multi['trans']}"] = NOE_trans.to_dict(orient="index")
# Deal with ambigous NOEs
NOE_trans = NOE_trans.explode("md")
# and ambigous/multiple values
NOE_trans = NOE_trans.explode("NMR exp")
fig, axs[0] = src.noe.plot_NOE(NOE_trans, fig, axs[0])
axs[0].set_title(f"Compound {multi['trans']} (trans)")
else:
print("Trans skipped because no frames are cis")
else:
NOE_dict = NOE.to_dict(orient="index")
NOE["md"], _, _2, NOE_dist, _3 = src.noe.compute_NOE_mdtraj(NOE_dict, t)
# Save NOE dict
NOE_output = {f"{compound_index}": NOE.to_dict(orient="index")}
# Deal with ambigous NOEs
NOE = NOE.explode("md")
# and ambigous/multiple values
NOE = NOE.explode("NMR exp")
fig, ax = src.noe.plot_NOE(NOE)
ax.set_title(f"Compound {compound_index}. NOE without reweighting.", y=1.2)
fig.tight_layout()
fig.savefig(snakemake.output.noe_plot, dpi=DPI)
# save as .json file
src.utils.json_dump(snakemake.output.noe_result, NOE_output)
2.22.6.2. Reweighted NOEs#
The following NOE plot was reweighted via a 1d PMF method.
# 1d PMF reweighted NOEs
NOE_output = {}
if snakemake.params.method != "cMD":
if multiple:
fig, axs = plt.subplots(2, 1, figsize=(6.7323, 6.7323))
NOE_trans, NOE_cis = NOE
NOE_cis_dict = NOE_cis.to_dict(orient="index")
NOE_trans_dict = NOE_trans.to_dict(orient="index")
if len(cis) > CIS_TRANS_CUTOFF:
(
NOE_cis["md"],
NOE_cis["lower"],
NOE_cis["upper"],
NOE_dist_cis,
pmf_plot_cis,
) = src.noe.compute_NOE_mdtraj(
NOE_cis_dict, t[cis],
reweigh_type=1, slicer=cis, weight_data=weight_data,
)
# TODO: this should not give an error!
NOE_output[f"{multi['cis']}"] = NOE_cis.to_dict(orient="index")
# Deal with ambigous NOEs
NOE_cis = NOE_cis.explode(["md", "lower", "upper"])
# and ambigous/multiple values
NOE_cis = NOE_cis.explode("NMR exp")
fig, axs[1] = src.noe.plot_NOE(NOE_cis, fig, axs[1])
axs[1].set_title(f"Compound {multi['cis']} (cis)")
else:
print("Cis skipped because no frames are cis.")
if len(trans) > CIS_TRANS_CUTOFF:
(
NOE_trans["md"],
NOE_trans["lower"],
NOE_trans["upper"],
NOE_dist_trans,
pmf_plot_trans,
) = src.noe.compute_NOE_mdtraj(
NOE_trans_dict, t[trans],
reweigh_type=1, slicer=trans, weight_data=weight_data
)
NOE_output[f"{multi['trans']}"] = NOE_trans.to_dict(orient="index")
# Deal with ambigous NOEs
NOE_trans = NOE_trans.explode(["md", "lower", "upper"])
# and ambigous/multiple values
NOE_trans = NOE_trans.explode("NMR exp")
fig, axs[0] = src.noe.plot_NOE(NOE_trans, fig, axs[0])
axs[0].set_title(f"Compound {multi['trans']} (trans)")
else:
print("Trans skipped because no frames are cis")
src.utils.json_dump(snakemake.output.noe_result, NOE_output)
fig.tight_layout()
fig.savefig(snakemake.output.noe_plot)
else:
NOE = src.noe.read_NOE(snakemake.input.noe)
NOE_dict = NOE.to_dict(orient="index")
NOE["md"], NOE["lower"], NOE["upper"], _, pmf_plot = src.noe.compute_NOE_mdtraj(
NOE_dict, t, reweigh_type=1, weight_data=weight_data
)
plt.close()
# Save NOE dict
NOE_output = {f"{compound_index}": NOE.to_dict(orient="index")}
# save as .json file
src.utils.json_dump(snakemake.output.noe_result, NOE_output)
# Deal with ambigous NOEs
NOE = NOE.explode(["md", "lower", "upper"])
# and ambigous/multiple values
NOE = NOE.explode("NMR exp")
fig, ax = src.noe.plot_NOE(NOE)
# ax.set_title(f"Compound {compound_index}. NOE", y=1.5, pad=0)
fig.tight_layout()
fig.savefig(snakemake.output.noe_plot, dpi=DPI)
else:
print("cMD - no reweighted NOEs performed.")
final_figure_axs.append(sg.from_mpl(fig))
pickle_dump(snakemake.output.noe_dist, NOE_dist)
cMD - no reweighted NOEs performed.
display(NOE)
| Atom 1 | Atom 2 | NMR exp | lower bound | upper bound | md | |
|---|---|---|---|---|---|---|
| 0 | (5,) | (3,) | 3.2 | 2.0 | 4.4 | 2.520337 |
| 1 | (5,) | (1,) | 3.2 | 2.0 | 4.4 | 3.107238 |
| 2 | (17,) | (15,) | 3.2 | 2.0 | 4.4 | 2.925682 |
| 3 | (39,) | (15,) | 3.2 | 2.0 | 4.4 | 4.93493 |
| 4 | (39,) | (37,) | 3.2 | 2.0 | 4.4 | 2.930614 |
| ... | ... | ... | ... | ... | ... | ... |
| 70 | (92, 93) | (95, 96) | 4.5 | 2.9 | 6.1 | 2.324332 |
| 71 | (92, 93) | (95, 96) | 3.5 | 2.3 | 4.7 | 2.330533 |
| 71 | (92, 93) | (95, 96) | 3.5 | 2.3 | 4.7 | 2.835695 |
| 71 | (92, 93) | (95, 96) | 3.5 | 2.3 | 4.7 | 2.803285 |
| 71 | (92, 93) | (95, 96) | 3.5 | 2.3 | 4.7 | 2.324332 |
177 rows × 6 columns
# matplotlib.rcParams.update(matplotlib.rcParamsDefault)
if snakemake.params.method != "cMD":
if not multiple:
pmf_plot.suptitle("NOE PMF plots")
pmf_plot.tight_layout()
pmf_plot.savefig(snakemake.output.noe_pmf)
fig = pmf_plot
else:
# save to image data
io_cis = io.BytesIO()
io_trans = io.BytesIO()
if len(cis) > CIS_TRANS_CUTOFF:
pmf_plot_cis.savefig(io_cis, format="raw", dpi=pmf_plot_cis.dpi)
if len(trans) > CIS_TRANS_CUTOFF:
pmf_plot_trans.savefig(io_trans, format="raw", dpi=pmf_plot_trans.dpi)
if len(cis) > CIS_TRANS_CUTOFF:
io_cis.seek(0)
img_cis = np.reshape(
np.frombuffer(io_cis.getvalue(), dtype=np.uint8),
newshape=(
int(pmf_plot_cis.bbox.bounds[3]),
int(pmf_plot_cis.bbox.bounds[2]),
-1,
),
)
io_cis.close()
if len(trans) > CIS_TRANS_CUTOFF:
io_trans.seek(0)
img_trans = np.reshape(
np.frombuffer(io_trans.getvalue(), dtype=np.uint8),
newshape=(
int(pmf_plot_trans.bbox.bounds[3]),
int(pmf_plot_trans.bbox.bounds[2]),
-1,
),
)
io_trans.close()
fig, axs = plt.subplots(2, 1)
fig.set_size_inches(16, 30)
if len(cis) > CIS_TRANS_CUTOFF:
axs[0].imshow(img_cis)
axs[0].axis("off")
axs[0].set_title("cis")
if len(trans) > CIS_TRANS_CUTOFF:
axs[1].imshow(img_trans)
axs[1].set_title("trans")
axs[1].axis("off")
# fig.suptitle('PMF plots. PMF vs. distance')
fig.tight_layout()
fig.savefig(snakemake.output.noe_pmf, dpi=DPI)
else:
fig, ax = plt.subplots()
ax.text(0.5, 0.5, "not applicable.")
fig.savefig(snakemake.output.noe_pmf, dpi=DPI)
display(fig)
2.22.7. NOE-Statistics#
Following, we compute various statistical metrics to evaluate how the simulated NOEs compare to the experimental ones.
# Compute deviations of experimental NOE values to the MD computed ones
NOE_stats_keys = []
NOE_i = []
NOE_dev = {}
if multiple:
if len(cis) > CIS_TRANS_CUTOFF:
NOE_stats_keys.append("cis")
NOE_i.append(NOE_cis)
if len(trans) > CIS_TRANS_CUTOFF:
NOE_stats_keys.append("trans")
NOE_i.append(NOE_trans)
else:
NOE_stats_keys.append("single")
NOE_i.append(NOE)
for k, NOE_d in zip(NOE_stats_keys, NOE_i):
if (NOE_d["NMR exp"].to_numpy() == 0).all():
# if all exp values are 0: take middle between upper / lower bound as reference value
NOE_d["NMR exp"] = (NOE_d["upper bound"] + NOE_d["lower bound"]) * 0.5
# Remove duplicate values (keep value closest to experimental value)
NOE_d["dev"] = NOE_d["md"] - np.abs(NOE_d["NMR exp"])
NOE_d["abs_dev"] = np.abs(NOE_d["md"] - np.abs(NOE_d["NMR exp"]))
NOE_d = NOE_d.sort_values("abs_dev", ascending=True)
NOE_d.index = NOE_d.index.astype(int)
NOE_d = NOE_d[~NOE_d.index.duplicated(keep="first")].sort_index(kind="mergesort")
NOE_d = NOE_d.dropna()
NOE_dev[k] = NOE_d
# Compute NOE statistics
NOE_stats = {}
for k in NOE_stats_keys:
NOE_d = NOE_dev[k]
NOE_stats_k = pd.DataFrame(columns=["stat", "value", "up", "low"])
MAE, upper, lower = src.stats.compute_MAE(NOE_d["NMR exp"], NOE_d["md"])
append = {"stat": "MAE", "value": MAE, "up": upper, "low": lower}
NOE_stats_k = NOE_stats_k.append(append, ignore_index=True)
MSE, upper, lower = src.stats.compute_MSE(NOE_d["dev"])
append = {"stat": "MSE", "value": MSE, "up": upper, "low": lower}
NOE_stats_k = NOE_stats_k.append(append, ignore_index=True)
RMSD, upper, lower = src.stats.compute_RMSD(NOE_d["NMR exp"], NOE_d["md"])
append = {"stat": "RMSD", "value": RMSD, "up": upper, "low": lower}
NOE_stats_k = NOE_stats_k.append(append, ignore_index=True)
pearsonr, upper, lower = src.stats.compute_pearsonr(NOE_d["NMR exp"], NOE_d["md"])
append = {"stat": "pearsonr", "value": pearsonr, "up": upper, "low": lower}
NOE_stats_k = NOE_stats_k.append(append, ignore_index=True)
kendalltau, upper, lower = src.stats.compute_kendalltau(
NOE_d["NMR exp"], NOE_d["md"]
)
append = {"stat": "kendalltau", "value": kendalltau, "up": upper, "low": lower}
NOE_stats_k = NOE_stats_k.append(append, ignore_index=True)
chisq, upper, lower = src.stats.compute_chisquared(NOE_d["NMR exp"], NOE_d["md"])
append = {"stat": "chisq", "value": chisq, "up": upper, "low": lower}
NOE_stats_k = NOE_stats_k.append(append, ignore_index=True)
fulfilled = src.stats.compute_fulfilled_percentage(NOE_d)
append = {"stat": "percentage_fulfilled", "value": fulfilled, "up": 0, "low": 0}
NOE_stats_k = NOE_stats_k.append(append, ignore_index=True)
NOE_stats[k] = NOE_stats_k
# Compute statistics for most populated cluster
if multiple:
NOE_stats_keys = ["cis", "trans"]
differentiation = {"cis": cis, "trans": trans}
else:
NOE_stats_keys = ["single"]
n_cluster_traj = {}
n_cluster_percentage = {}
n_cluster_index = {}
remover = []
for k in NOE_stats_keys:
if multiple:
cluster_in_x = np.in1d(cluster_index, differentiation[k])
print(cluster_in_x)
if np.all(cluster_in_x == False):
# No clusters found for specific cis/trans/other
remover.append(k)
else:
cluster_in_x = np.ones((len(cluster_index)), dtype=bool)
cluster_in_x = np.arange(0, len(cluster_index))[cluster_in_x]
n_cluster_traj[k] = cluster_traj[cluster_in_x]
n_cluster_percentage[k] = np.array(cluster_percentage)[cluster_in_x]
n_cluster_index[k] = np.array(cluster_index)[cluster_in_x]
cluster_traj = n_cluster_traj
cluster_percentage = n_cluster_percentage
cluster_index = n_cluster_index
[NOE_stats_keys.remove(k) for k in remover]
[]
# Compute statistics for most populated cluster
NOE_dict = {}
NOE = src.noe.read_NOE(snakemake.input.noe)
NOE_n = {}
if multiple:
NOE_trans, NOE_cis = NOE
NOE_n["cis"] = NOE_cis
NOE_n["trans"] = NOE_trans
NOE_dict["cis"] = NOE_cis.to_dict(orient="index")
NOE_dict["trans"] = NOE_trans.to_dict(orient="index")
else:
NOE_dict["single"] = NOE.to_dict(orient="index")
NOE_n["single"] = NOE
for k in NOE_stats_keys:
# max. populated cluster
# NOE = NOE_n.copy()
max_populated_cluster_idx = np.argmax(cluster_percentage[k])
max_populated_cluster = cluster_traj[k][max_populated_cluster_idx]
NOE_n[k]["md"], *_ = src.noe.compute_NOE_mdtraj(NOE_dict[k], max_populated_cluster)
# Deal with ambigous NOEs
NOE_n[k] = NOE_n[k].explode("md")
# and ambigous/multiple values
NOE_n[k] = NOE_n[k].explode("NMR exp")
# Remove duplicate values (keep value closest to experimental value)
NOE_test = NOE_n[k]
if (NOE_test["NMR exp"].to_numpy() == 0).all():
# if all exp values are 0: take middle between upper / lower bound as reference value
NOE_test["NMR exp"] = (NOE_test["upper bound"] + NOE_test["lower bound"]) * 0.5
NOE_test["dev"] = NOE_test["md"] - np.abs(NOE_test["NMR exp"])
NOE_test["abs_dev"] = np.abs(NOE_test["md"] - np.abs(NOE_test["NMR exp"]))
NOE_test = NOE_test.sort_values("abs_dev", ascending=True)
NOE_test.index = NOE_test.index.astype(int)
NOE_test = NOE_test[~NOE_test.index.duplicated(keep="first")].sort_index(
kind="mergesort"
)
# drop NaN values:
NOE_test = NOE_test.dropna()
# Compute metrics now
# Compute NOE statistics, since no bootstrap necessary, do a single iteration.. TODO: could clean this up further to pass 0, then just return the value...
RMSD, *_ = src.stats.compute_RMSD(
NOE_test["NMR exp"], NOE_test["md"], n_bootstrap=1
)
MAE, *_ = src.stats.compute_MAE(NOE_test["NMR exp"], NOE_test["md"], n_bootstrap=1)
MSE, *_ = src.stats.compute_MSE(NOE_test["dev"], n_bootstrap=1)
fulfil = src.stats.compute_fulfilled_percentage(NOE_test)
# insert values
values = [MAE, MSE, RMSD, None, None, None, fulfil]
NOE_stats[k].insert(4, "most-populated-1", values)
# If there are no cis/trans clusters, still write a column 'most-populated-1', but fill with NaN
for k in remover:
values = [np.nan, np.nan, np.nan, np.nan, np.nan, np.nan, np.nan]
NOE_stats[k].insert(4, "most-populated-1", values)
for k in NOE_stats.keys():
display(NOE_stats[k])
# convert df to dict for export
NOE_stats[k] = NOE_stats[k].to_dict()
# Save
src.utils.json_dump(snakemake.output.noe_stats, NOE_stats)
| stat | value | up | low | most-populated-1 | |
|---|---|---|---|---|---|
| 0 | MAE | 0.881346 | 1.004304 | 0.758732 | 0.662635 |
| 1 | MSE | -0.655128 | -0.466760 | 0.000000 | -0.230779 |
| 2 | RMSD | 1.027598 | 1.137252 | 0.908837 | 0.998567 |
| 3 | pearsonr | 0.422412 | 0.571881 | 0.249060 | NaN |
| 4 | kendalltau | 0.477305 | 0.595163 | 0.321566 | NaN |
| 5 | chisq | 17.522493 | 21.494913 | 13.793357 | NaN |
| 6 | percentage_fulfilled | 0.902778 | 0.000000 | 0.000000 | 0.958333 |
plt.rc('font', size=MEDIUM_SIZE) # controls default text sizes
plt.rc('axes', titlesize=BIGGER_SIZE) # fontsize of the axes title
plt.rc('axes', labelsize=MEDIUM_SIZE) # fontsize of the x and y labels
plt.rc('xtick', labelsize=MEDIUM_SIZE) # fontsize of the tick labels
plt.rc('ytick', labelsize=MEDIUM_SIZE) # fontsize of the tick labels
plt.rc('legend', fontsize=MEDIUM_SIZE) # legend fontsize
plt.rc('figure', titlesize=BIGGER_SIZE) # fontsize of the figure title
if multiple:
fig, axs = plt.subplots(2, 1)
if len(cis) > CIS_TRANS_CUTOFF:
# cis
axs[0].scatter(NOE_dev["cis"]["NMR exp"], NOE_dev["cis"]["md"])
axs[0].set_ylabel("MD")
axs[0].set_xlabel("Experimental NOE value")
axs[0].axline((1.5, 1.5), slope=1, color="black")
axs[0].set_title("Experimental vs MD derived NOE values - cis")
if len(trans) > CIS_TRANS_CUTOFF:
# trans
axs[1].scatter(NOE_dev["trans"]["NMR exp"], NOE_dev["trans"]["md"])
axs[1].set_ylabel("MD")
axs[1].set_xlabel("Experimental NOE value")
axs[1].axline((1.5, 1.5), slope=1, color="black")
axs[1].set_title("Experimental vs MD derived NOE values - trans")
fig.tight_layout()
fig.savefig(snakemake.output.noe_stat_plot)
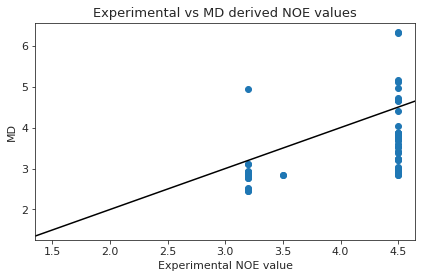
else:
plt.scatter(NOE_dev["single"]["NMR exp"], NOE_dev["single"]["md"])
if snakemake.params.method != "cMD":
plt.scatter(
NOE_dev["single"]["NMR exp"], NOE_dev["single"]["upper"], marker="_"
)
plt.scatter(
NOE_dev["single"]["NMR exp"], NOE_dev["single"]["lower"], marker="_"
)
plt.ylabel("MD")
plt.xlabel("Experimental NOE value")
plt.axline((1.5, 1.5), slope=1, color="black")
plt.title("Experimental vs MD derived NOE values")
plt.tight_layout()
plt.savefig(snakemake.output.noe_stat_plot)
# is the mean deviation significantly different than 0? if pvalue < 5% -> yes! We want: no! (does not deviate from exp. values)
if multiple:
if len(cis) > CIS_TRANS_CUTOFF:
print(stats.ttest_1samp(NOE_dev["cis"]["dev"], 0.0))
if len(trans) > CIS_TRANS_CUTOFF:
print(stats.ttest_1samp(NOE_dev["trans"]["dev"], 0.0))
else:
print(stats.ttest_1samp(NOE_dev["single"]["dev"], 0.0))
Ttest_1sampResult(statistic=-6.972737087357061, pvalue=1.317449708175049e-09)
if multiple:
if len(cis) > CIS_TRANS_CUTOFF:
print(stats.describe(NOE_dev["cis"]["dev"]))
if len(trans) > CIS_TRANS_CUTOFF:
print(stats.describe(NOE_dev["trans"]["dev"]))
else:
print(stats.describe(NOE_dev["single"]["dev"]))
DescribeResult(nobs=72, minmax=(-1.6643049482285228, 1.8247033988384933), mean=-0.655128135049046, variance=0.6355919941216246, skewness=1.1187931275707077, kurtosis=1.608813690137989)
# Make overview figure
plot1 = final_figure_axs[0].getroot()
plot2 = final_figure_axs[1].getroot()
plot3 = final_figure_axs[2].getroot()
plot4 = final_figure_axs[3].getroot()
plot5 = final_figure_axs[4].getroot()
if multiple:
# # TODO: fix this!
# sc.Figure(
# "4039",
# "5048",
# sc.Panel(plot3, sc.Text("A", 0, 0, size=16, weight='bold')),
# sc.Panel(
# plot2,
# sc.Text("B", "8.5cm", "0cm", size=16),
# ).move(0,200),
# sc.Panel(plot4, sc.Text("C", 25, 20, size=16, weight='bold')),
# # sc.Panel(plot4, sc.Text("D", 25, 20, size=20, weight='bold')).scale(0.8).move(-200,0),
# ).tile(2, 2).save(snakemake.output.overview_plot)
sc.Figure(
"4039",
"7068", # 5048
sc.Panel(plot2, sc.Text("A", 25, 20, size=16, weight='bold').move(-12,0)).scale(8),
sc.Panel(plot3, sc.Text("B", 25, 20, size=16, weight='bold').move(-12,0)).scale(8),
sc.Panel(plot4, sc.Text("C", 25, 20, size=16, weight='bold').move(-12,-24)).scale(8).move(0, -300),
sc.Panel(sc.Text("2")),
sc.Panel(plot5, sc.Text("D", 25, 20, size=16, weight='bold').move(-12,0)).scale(8).move(0, -1550),
# sc.Panel(plot1, sc.Text("D", 25, 0, size=16, weight='bold')),
).tile(2, 3).save(snakemake.output.overview_plot)
else:
sc.Figure(
"4039",
"5048", # 4039
sc.Panel(plot2, sc.Text("A", 25, 20, size=16, weight='bold').move(-12,0)).scale(8),
sc.Panel(plot3, sc.Text("B", 25, 20, size=16, weight='bold').move(-12,0)).scale(8),
sc.Panel(plot4, sc.Text("C", 25, 20, size=16, weight='bold').move(-12,-24)).scale(8).move(0, 350),
sc.Panel(sc.Text("2")),
sc.Panel(plot5, sc.Text("D", 25, 20, size=16, weight='bold').move(-12,0)).scale(8).move(0, -250),
# sc.Panel(plot1, sc.Text("D", 25, 0, size=16, weight='bold')),
).tile(2, 3).save(snakemake.output.overview_plot)
src.utils.show_svg(snakemake.output.overview_plot)
print("Done")
Done
